Contracaecum osculatum larvae in Baltic cod (Gadus morhua): Effects on growth and immune response
Contracaecum osculatum larvae in Baltic cod (Gadus morhua): Effects on growth and immune response
PhD Student: Huria Marnis email huria.marnis@sund.ku.dk
Department of Veterinary and Animal Sciences, section for Parasitology and Aquatic Pathobiology
Thesis defended: 6 May 2021
The eastern Baltic cod has experienced a marked increase in infection caused by third-stage larvae of the nematode Contracaecum osculatum. The cod acts as transport host with the third-stage larvae located in the liver. The PhD project produced three published papers.
The first describes the distribution of the parasites in the cod population and presents indications for inter- and intraspecific interactions. Intraspecific competition between larvae in the cod liver was suggested by a decrease in the average length of worms associated with a high worm density. The interspecific interaction was shown by a low titer antibody response in cod against the excretory/secretory compounds produced by third-stage larvae. The cod immune response in liver and spleen was further investigated in the second paper applying a quantitative real-time PCR evaluating immune gene expression in infected and non-infected cod. Some immune genes were downregulated in the liver of infected cod while these genes in spleen from the same fish were upregulated, suggesting a parasite-induced immune suppression locally in the liver. The third paper is based on a comparative transcriptomic analysis of cod liver infected or non-infected by C. osculatum third-stage larvae. Effects of the parasite infection were indicated through a significant modulation of genes in cod. The changes were associated with host immune response, metabolism, and growth.
Purpose
The present Ph.D. project is a contribution to a better understanding of the complex interactions between helminth parasites and host animals in general and between C. osculatum larvae and cod in particular. The work has dissected and displayed some of the mechanisms involved in a parasite-induced malfunction of Baltic cod infected by C. osculatum parasites. The described depressing effects of the parasite infection on immune functions, physiological processes, and growth regulation in Baltic cod may contribute to an understanding of the current Baltic cod crisis.
Results
The distribution in the number of C. osculatum third-stage larvae in Baltic cod could be described by the negative binomial distribution, which was the distribution with the highest log-likelihood. The average observed number of parasites was 29.3 parasites per host (range 1-377) with the prevalence of infection 89.8 %, and a variance/mean ratio of 59.2 (>>1). Quantitative RT-PCR is the most extensively used approach for gene expression studies. Liver samples from infected fish were analyzed and classified as having a low, medium or a high infection. The overall percentage of samples reaching a Cq value was 90.22% in liver tissue compared to 98.88% in spleen tissue. Overall, 12 (52.17%) genes and 14 (60.86%) genes showed significant regulation in the liver and the spleen, respectively. A significant up-regulation of the gene expression in liver of cytokines associated with inflammatory reactions (il-1β, il-6, il-8) and significant down-regulation of genes encoding effector molecules (crp-p2, hepcidin, lysozyme g1, lysozyme g2, c3, and igdm), contributing to the elimination of pathogens in general, were found. Although several studies have reported activation of several immune genes in the infected cod, the immune response picture is still considered incomplete. To address this issue, a comparative transcriptomic analysis was performed on infected versus uninfected Baltic cod liver, and it showed that a total of 2084 (4.43%) out of 47,025 identified gene sequences were significantly regulated after parasite infection. Of the mentioned differentially expressed gene sequences (DEGs), 1240 genes were up-regulated, and 844 genes were down-regulated.
Perspectives
1. We realize that genetic variation between even closely related cod stocks and environmental differences between host groups may influence expression analyses and transcriptomic results. We used infected cod captured east of the island Bornholm and this was a natural infection (non-experimental infection). We collected non-infected control cod from a different location (Zealand) because non-infected eastern Baltic cod are not available. The best way to gain more accurate gene expressions and transcriptomic results are through a controlled experimental infection with C.osculatum.
- Future studies should design relevant primer/probe sets and measure the expression of genes that were left unstudied in the present Ph.D. work.
- Most of the immune genes studied were found downregulated in the cod liver and spleen in this study, so we suggest to measure the expression of immune genes in the other lymphoid organs, including kidney and thymus in cod infected with C.osculatum.
- How the C.osculatum larvae migrate to the cod liver is poorly understood and still needs to be explored. We know that cod are infected by C.osculatum larvae by ingesting smaller fish, such as sprat (Sprattus sprattus) in the Baltic Sea. When eaten the larvae may move from the cod stomach, the intestine or pyloric caeca and then penetrate the wall to reach the portal veins or alternatively the body cavity before entering the liver. As a third possibility, the larvae could target the bile duct and migrate to the liver through the gallbladder. Detailed investigations concerning the migration of C.osculatum third-stage larva to the cod liver may be conducted by use of larvae labelled by various markers.
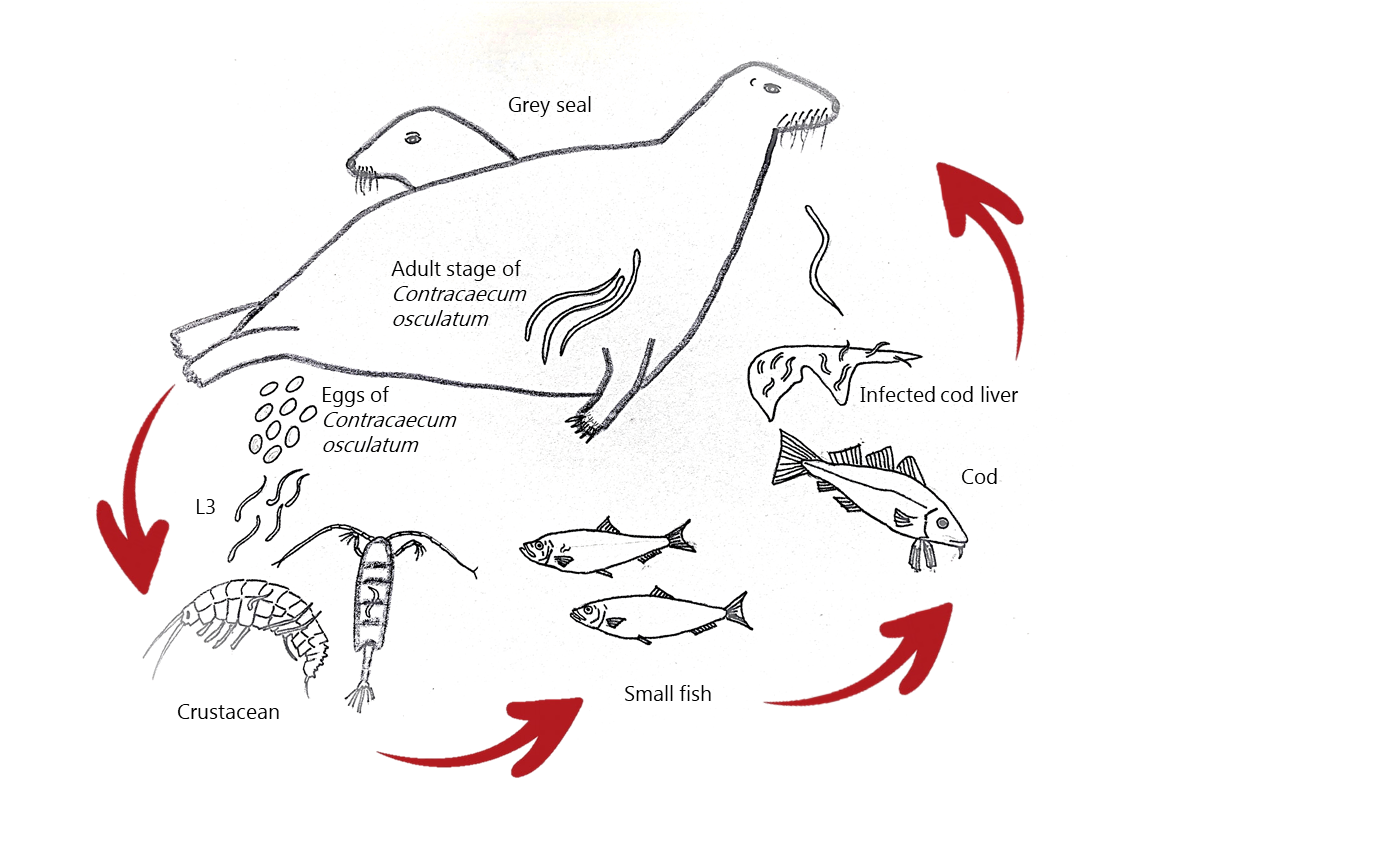
The life cycle of C. osculatum in the Baltic Sea. The adult female worm located in the seal stomach releases eggs. Original drawing by Kurt Buchmann.
