Immune Modulation by Human Milk Oligosaccharides
PhD Student: Stine Dam Jepsen
Background
Human milk oligosaccharides (HMOs), which are abundant in human milk, play a critical role in supporting infant health, particularly in promoting gut health and the development of a healthy microbiota. These complex carbohydrates, over 200 different structures in total, are categorized into three main groups: neutral, fucosylated, and sialylated. HMOs serve as a key nutritional component for infants, but they also have more recently gained attention for their potential immune-modulating properties.
Purpose
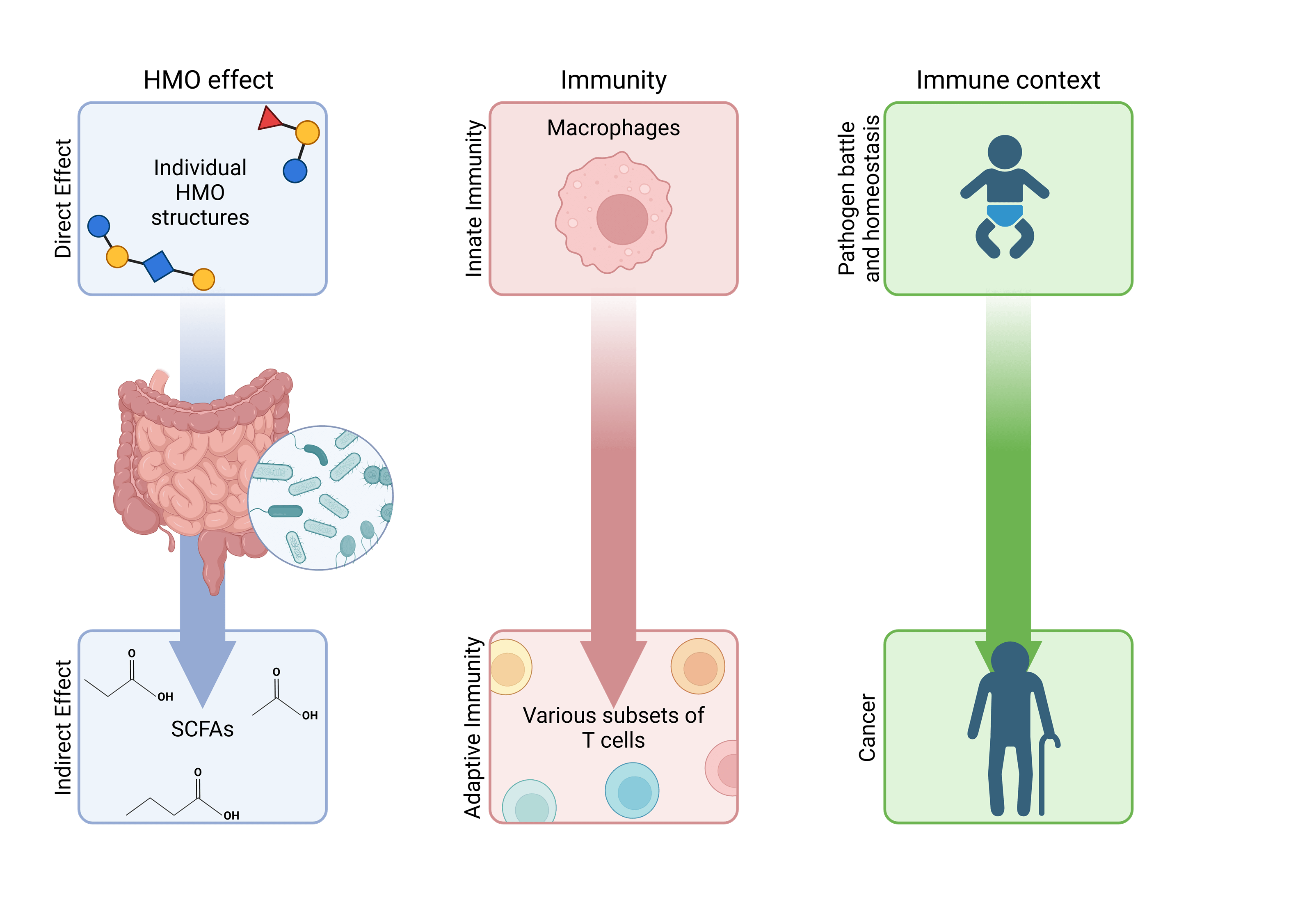
The overall aim of this thesis was to investigate how various structures of HMOs affect different parts of the immune system, both directly, independent of microbiota and microbial derives, as well as indirectly by involvement of HMO-fermentation products, in this case, short-chain fatty acids.
This thesis explores how different structures of human milk oligosaccharides (HMOs) can influence immune responses, expanding our understanding of their role in immune regulation.
Results
In the first part, the research demonstrates that three specific HMOs—6′SL, LNnT, and 2′FL—enhance immune responses against Staphylococcus aureus in macrophages. Among these, 6′SL was found to be the most effective. These HMOs triggered increased levels of M1-like markers, NF-κB activation, cell proliferation, and phagocytosis. Importantly, the immune responses were pathogen-dependent, meaning these HMOs can boost immunity without causing inflammation under normal, healthy conditions.
The second part of the study focused on how HMOs like 2′FL and 3FL influence adhesion molecules in T cells. The research found that 3FL, but not 2′FL, decreased the expression of LFA-1, a key adhesion molecule. This led to impaired T cell development and reduced activation and migration, which could have potential benefits in managing conditions with excessive immune responses, like allergies and autoimmune diseases.
Further, the thesis examined the effects of 2′FL and 3FL on cancer cells. It was found that 3FL, but not 2′FL, increased the expression of NKG2D ligands, which play a role in immune activation against cancer cells. The research also highlighted that propionate could enhance this effect in cancer cells, particularly in colorectal cancer cells.
In conclusion, this thesis emphasizes that the structure of HMOs, along with their fermentation products, can significantly impact immune responses, offering promising avenues for future research, particularly in immune-related diseases and cancer therapies.
Future perspectives
Despite the promising insights into the immune-modulating properties of human milk oligosaccharides (HMOs), the precise mechanisms through which different HMO structures influence immune responses remain inadequately understood. HMOs are structurally diverse, with variations such as neutral, fucosylated, and sialylated forms, suggesting that each type may interact with the immune system in distinct ways. Some studies indicate that specific HMOs may play a role in modulating various immune responses, however, the detailed molecular mechanisms underlying these effects, including the identification of relevant receptors, signaling pathways, and cellular interactions, remain to be fully elucidated. Additionally, the role of HMOs in modulating the gut microbiota and their impact on gut-associated immune responses warrant further investigation. As research progresses, a deeper understanding of how HMOs interact with the immune system and their modes of action will be crucial in exploring their potential as therapeutic agents. These insights could pave the way for novel treatment strategies for a range of immune-related conditions, from infectious diseases to autoimmune disorders and cancer, by harnessing the unique immunoregulatory properties of HMOs.